When a medical device company transitions from development and prototyping to full scale manufacturing, one of the most critical challenges is ensuring that all processes, equipment, and systems are ready to deliver consistent, safe, and high quality products. At this stage, the three core validation stages IQ, OQ, and PQ, become essential tools. These stages act as structured checkpoints not only for confirming compliance with regulations from authorities like the FDA, but also for minimizing risk, improving efficiency, and ensuring the final product meets all specifications.
What Are IQ, OQ, PQ and Why Are They Important in Scaling Production?
While IQ, OQ, and PQ are essential whenever new equipment or processes are introduced, they become especially important during scale-up. This phase often involves transitioning from R&D or pilot batches into full production, where validation ensures that the infrastructure is ready to meet regulatory expectations and production goals.
During the shift from pilot-scale production to commercial manufacturing, companies must ensure that every part of the production line functions properly, from the installation of equipment to its long term, consistent performance.
The IQ/OQ/PQ process provides a structured framework to verify that equipment is installed correctly (IQ), operates as intended (OQ), and consistently performs under real manufacturing conditions (PQ). Each phase builds confidence in the manufacturing process and is crucial for regulatory compliance, internal quality assurance, and long-term operational success.
IQ – Installation Qualification
IQ is the foundation of the validation process. It confirms that all manufacturing equipment and systems are installed according to design specifications, manufacturer requirements, and regulatory standards.
This phase typically includes:
- Verifying that equipment is located and anchored correctly per the approved layout
- Confirming all utility connections (e.g., electricity, compressed air, vacuum, water) are properly installed and operational
- Checking that installation follows cGMP standards and complies with safety and environmental requirements
- Ensuring all components and materials are correct, complete, and documented
- Reviewing calibration certificates for instruments, gauges, sensors, and control systems
Example: Installing a new cleanroom filling machine and confirming that all connections – electrical, air, and water – are properly made, materials meet cleanroom standards, and documentation is complete.
What does IQ verify?
IQ verifies the Design Specification – ensuring the system was built and installed as planned.
OQ – Operational Qualification
OQ focuses on verifying that the equipment and systems operate as expected within predefined operating ranges. It evaluates functionality, safety features, and control capabilities.
This phase typically includes:
- Running equipment at minimum, nominal, and maximum settings to verify reliable performance
- Validating critical alarms, interlocks, and emergency shutdown systems
- Simulating fault conditions to assess system responses
- Confirming automation and control logic (PLCs, HMIs) operate correctly
- Documenting all test results and deviations
Example: Running a packaging machine at different speeds to confirm seal temperature stability, testing alarms and interlocks, and ensuring it stops properly during a fault condition.
What does OQ verify?
OQ verifies the Functional Specification – confirming the system functions safely and reliably.
PQ – Performance Qualification
PQ is the final validation stage. It verifies that the equipment consistently produces products that meet quality specifications under actual production conditions.
This phase typically includes:
- Running multiple full-scale production batches (typically three) under routine conditions
- Sampling and testing finished products for critical quality attributes (CQA)
- Conducting statistical analysis (e.g., Cpk, PpK)
- Verifying documentation traceability and batch record completeness
Example: Producing three full batches of medical tubing under standard production conditions and confirming that all dimensional, mechanical, and visual specifications are consistently met.
What does PQ verify?
PQ verifies the User Requirements Specification – proving that the system meets the real world needs of the user.
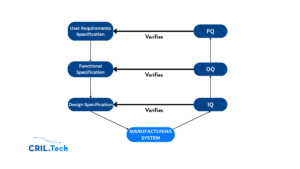
The System Validation Journey
CRIL Tech Support
At CRIL, we specialize in helping companies scale from early-stage production to full scale manufacturing. Whether setting up new production lines or validating new processes, we work closely with our clients to ensure each step is completed accurately and efficiently – IQ, OQ, or PQ
We closely monitor the entire validation journey, from installation to performance runs, ensuring that every requirement is met and documented. Our team works side by side with production, engineering, and quality personnel to address technical issues, streamline testing, and meet regulatory expectations without delays.
Our goal is to give our clients peace of mind during the scale up phase – delivering robust validation processes that ensure readiness for commercial success.
Planning to launch a new medical device production line?
Let’s talk about how CRIL can help you get there.
